The net movement of molecules from region of high concentration to regions of low concentration that is, down the concentration gradient. Water molecules in a solution are not static they are in continuous motion, colliding with one another and exchanging kinetic energy. The molecules intermingle as a result of their random thermal motion, this random motion is called diffusion. As long as other forces are not acting on the molecule, diffusion causes the net movement of molecules from region of high concentration to region of low concentration.
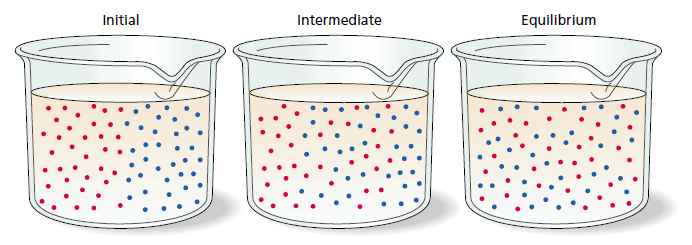
molecules and eventual dissipation of concentration differences. Initially, two materials
containing different molecules are brought into contact. The materials may be
gas, liquid, or solid. Diffusion is fastest in gases, slower in liquids, and slowest in
solids. The initial separation of the molecules is depicted graphically in the upper
panels, and the corresponding concentration profiles are shown in the lower panels
as a function of position. With time, the mixing and randomization of the molecules
diminishes net movement. At equilibrium the two types of molecules are randomly
(evenly) distributed.
SOME OF THE IMPORTANT FACTORS:
(A) Diffusion Pressure Gradient (DPG):
The rate of diffusion of any substance is directly proportional to the difference in concentration of its molecules or ions in the two regions, and inversely proportional to the distance between these two regions. Thus, the differences in diffusion pressures determine the rate and direction of diffusion.
(B) Temperature:
Temperature greatly influences the rate of diffusion. If the temperature is raised, diffusion is accelerated because the velocity of the diffusing particles is increased.
(C) Density:
Concentration of the diffusing particles and the density of the liquid or gas through which the diffusion occurs markedly influences the rate of diffusion. Density of the diffusing gas itself determines the rate of diffusion. Lighter the gas, greater will be the rate of its diffusion. According to the law of diffusion of gases, the rate of diffusion is inversely proportional to the square root of the density of gas.
Importance of diffusion in Plants:
1. The exchange of gases through stomata (for example, CO2 intake and O2 output during photosynthesis, and CO2 output and O2 intake during respiration takes place by the principal of independent diffusion.
2. Transpiration involves the process of diffusion.
3. The ions are absorbed by the simple diffusion.
4. Diffusion is an effective means of transport of substances helps in the translocation of food material.
5. Aroma in the vicinity of flowers is nothing but the diffusion of the volatile aromatic compounds. Thus, the diffusion helps to attract insects and other animals for pollination.
6. Diffusion keeps the cell walls of the internal plant tissues moist.
7. It is a means of spreading of ions and other substances throughout the protoplast.