We noted earlier that one of the principal functions of the respiration is to retrieve, in useful form, some of the energy initially stored in assimilates. Our traditional measure of useful energy in most processes is the number of ATP molecules gained or consumed. By this measure alone, the yield from both glycolysis and the citric acid cycle is quite low.
After two complete turns of the cycle, one molecule of glucose has been completely oxidized to six molecules of CO2, but only four molecules of ATP have been produced (a net of two ATP from glycolysis plus one for each turn of the cycle).
At this point, most of the energy associated with the glucose molecule has been conserved in the form of electron pairs generated by the oxidation of glycolytic and citric acid cycle intermediates.
For each molecule of glucose, a total of 12 electron pairs were generated;10 as NADH and two as FADH.
In this section we will discuss the third stage of cellular respiration—the transfer of electrons from NADH and FADH2 to oxygen and the accompanying conversion of redox energy to ATP. The transfer of electrons from NADH and FADH2 to oxygen involves a sequence of electron carriers arranged in an electron transport chain.
Complex-I
Electrons from NADH enter the electron transport chain through ComplexI, known as NADH-ubiquinone oxidoreductase.
In addition to several proteins, this complex also contains a tightly bound molecule of flavin mononucleotide (FMN) and several non heme iron-sulphur centers.
Complex I conveys the electrons from NADH to ubiquinone.
Complex III, or cytochrome c reductase.
Ubiquinol (the fully reduced form of ubiquinone) is oxidized by Complex III, or cytochrome c reductase.
Complex III contains cytochromes b and c1 and an iron-sulphur center.
Complex IV, cytochrome c oxidase
It contains cytochromes a and a3 and copper. Electrons are passed first from cytochrome c to cytochrome a, then to cytochrome a3, and finally to molecular oxygen.
All of the oxidative enzymes of the citric acid cycle, with one exception, are located in the matrix. The one exception is succinic dehydrogenase. This enzyme is an integral protein complex (Complex II) that is tightly bound to the inner mitochondrial membrane.
Infact, succinic dehydrogenase is the preferred marker enzyme for inner membranes when doing mitochondrial fractionations.
ComplexII
ComplexII,known as succinate-ubiquinone oxidoreductase, contains flavin adenine di-nucleotide (FAD), several non heme iron proteins, and iron-sulphur centers.
Like Complex I, succinic dehydrogenase transfers electrons from succinate to a molecule of ubiquinone from the membrane pool. From there the electrons pass through Complexes III and IV to molecular oxygen.
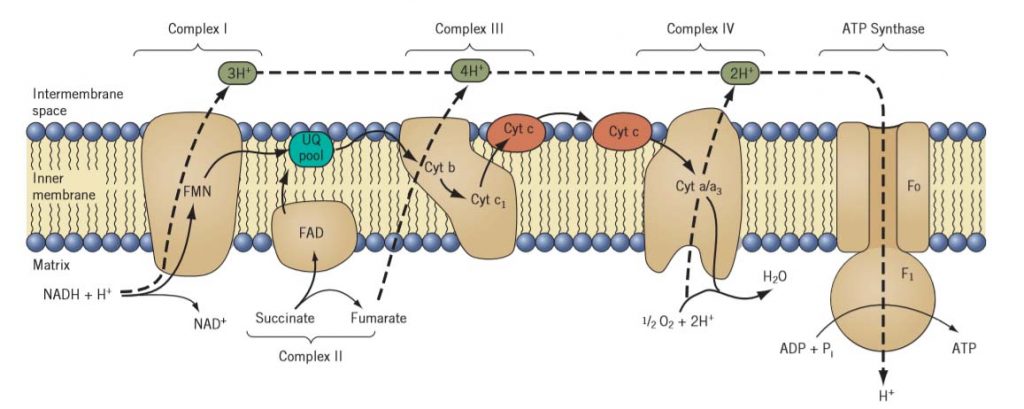
Fig: A schematic representation of the electron transport chain and proton ‘‘pumping’’ sites in the inner membrane of a plant mitochondrion. Solid arrow indicates the path of electrons from NADH or succinate to molecular oxygen. Energy conserved in the proton gradient is used to drive ATP synthesis through the F0—F1-ATPase coupling factor elsewhere in the membrane.
ENERGY IS CONSERVED IN THE FORM OF ATP IN ACCORDANCE WITH CHEMIOSMOSIS
As electrons are passed from NADH (or FADH2) to oxygen through the electron transport chain, there is a substantial drop in free energy.
Because mitochondrial ATP synthesis is closely tied to oxygen consumption, it is referred to as oxidative phosphorylation.