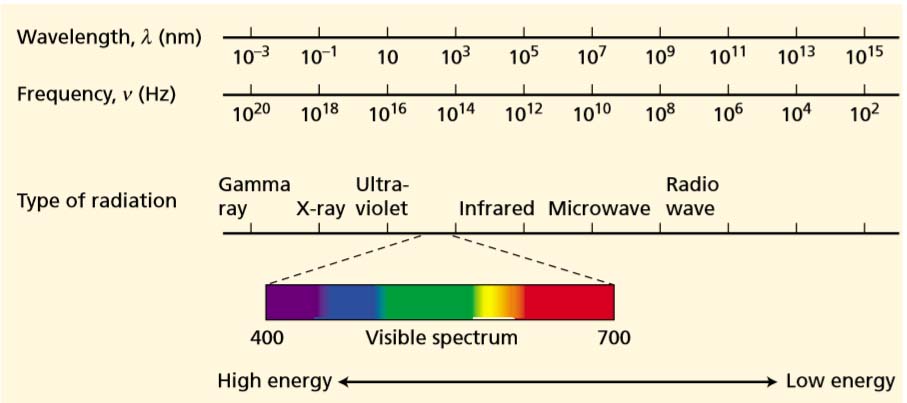
Light is the visible portion of spectrum.
Light Has Characteristics of Both a Particle and a Wave
A wave is characterized by a wavelength, denoted by the Greek letter lambda ( ƛ ), which is the distance between successive wave crests. The frequency, represented by the Greek letter nu ( v ), is the number of wave crests that pass an observer in a given time. A simple equation relates the wavelength, the frequency, and the speed of any wave: c = ƛ v
where c is the speed of the wave—in the present case, the speed of light (3.0 × 108 m s–1).
Light is also a particle, which we call a photon. Each photon contains an amount of energy that is called a quantum(plural quanta). The energy content of light is not continuous but rather is delivered in these discrete packets, the quanta. The energy (E) of a photon depends on the frequency of the light according to a relation known as Planck’s law:
E = hv
Where h is Plank’s constant.
Sunlight is like a rain of photons of different frequencies.
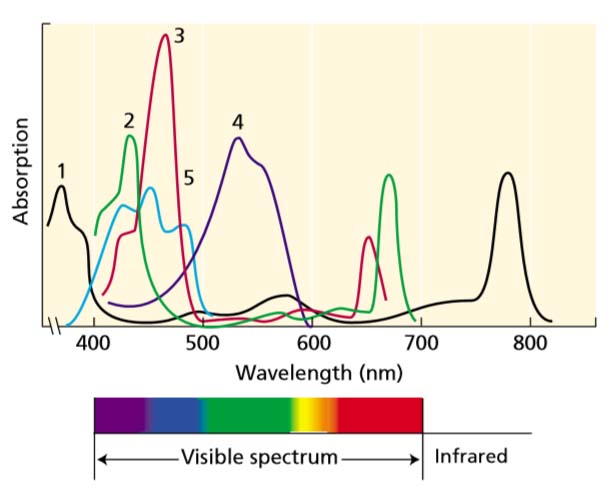
Absorption Spectrum
The portion of visible spectrum absorbed by pigments (e.g., chlorophyll) is called absorption spectrum.
Chlorophyll a: show maximum absorption in the violet-blue and red region.
Chlorophyll b: Also shows maximum absorption in blue and red regions but slightly different wavelengths than chlorophyll a.
Action spectrum
| Quantum Yield; number of oxygen molecules released per quantum of light absorbed- this number is usually 1/8 or 12% |
A plot showing relative effectiveness of different wavelengths of light in photosynthesis is called action spectrum. It is the measure of effect of absorbed light. When we compare the effect of different wavelengths on the rate of photosynthesis, we obtain an action spectrum.
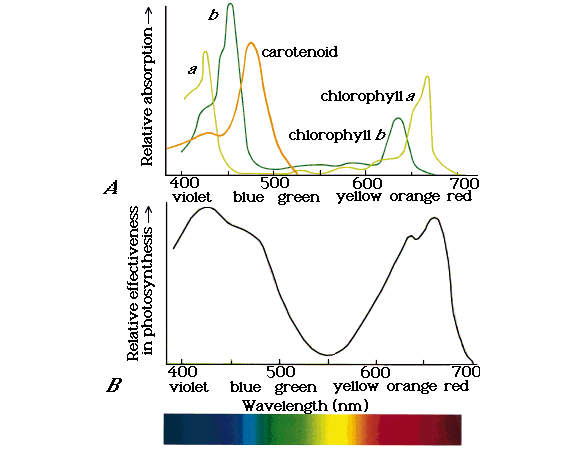
action spectrum always exceeds the absorption spectrum