Water makes up most of the mass of plant cells. Each cell contains a large water-filled vacuole. In such cells the cytoplasm makes up only 5 to 10% of the cell volume; the remainder is vacuole. Water typically constitutes 80 to 95% of the mass of growing plant tissues. Common vegetables such as carrots and lettuce may contain 85 to 95% water. Wood, which is composed mostly of dead cells, has a lower water content; sapwood, which functions in transport in the xylem, contains 35 to 75% water; and heartwood has a slightly lower water content.
Seeds, with a water content of 5 to 15%, are among the driest of plant tissues, yet before germinating they must absorb a considerable amount of water.
Structure of water
Water has special properties that enable it to act as a solvent and to be readily transported through the body of the plant. These properties derive primarily from the polar structure of the water molecule. In this section we will examine how the formation of hydrogen bonds contributes to the properties of water that are necessary for life.
Polarity of Water
The water molecule consists of an oxygen atom covalently bonded to two hydrogen atoms. The two O—H bonds form an angle of 105°. Because the oxygen atom is more electronegative than hydrogen, it tends to attract the electrons of the covalent bond. This attraction results in a partial negative charge at the oxygen end of the molecule and a partial positive charge at each hydrogen.
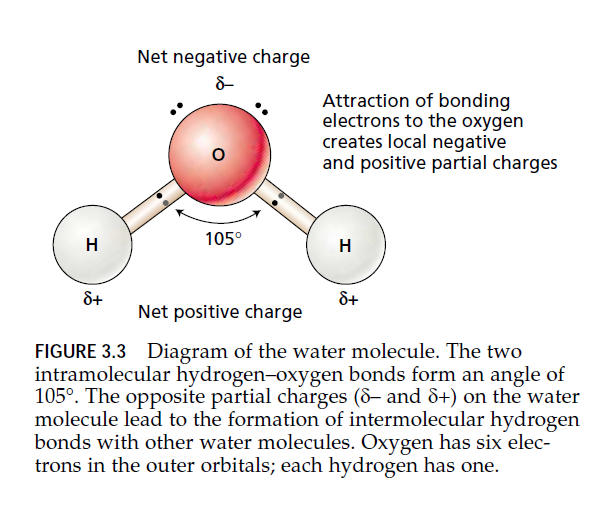
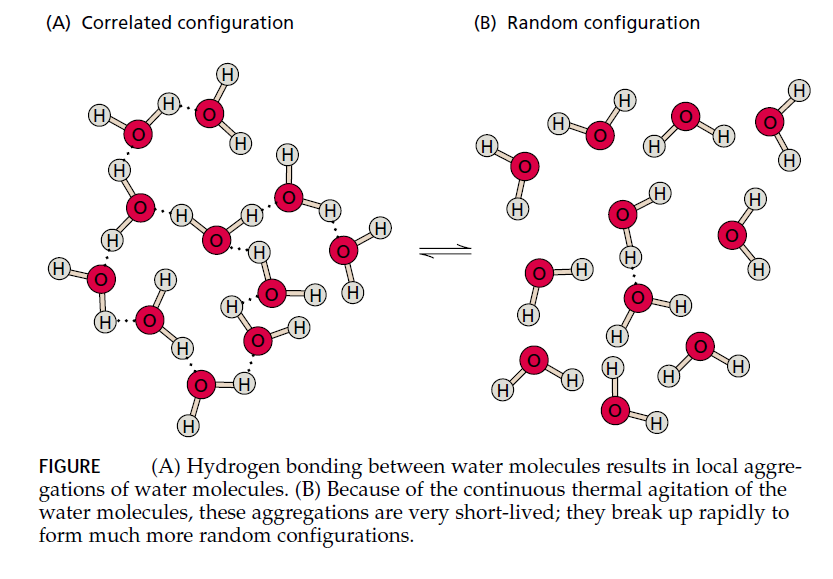
This separation of partial charges, together with the shape of the water molecule, makes water a polar molecule, and the opposite partial charges between neighboring water molecules tend to attract each other. The weak electrostatic attraction between water molecules, known as a hydrogen bond, is responsible for many of the unusual physical properties of water.
Water; Universal solvent
Water is an excellent solvent: It dissolves greater amounts of a wider variety of substances than do other related solvents. This versatility as a solvent is due in part to the small size of the water molecule and in part to its polar nature.
The latter makes water a particularly good solvent for ionic substances and for molecules such as sugars and proteins that contain polar —OH or —NH2 groups.
Thermal Properties of Water
Specific heat
is the heat energy required to raise the temperature of a substance by a specific amount.
When the temperature of water is raised, the molecules vibrate faster and with greater amplitude. To allow for this motion, energy must be added to the system to break the hydrogen bonds between water molecules. Thus, compared with other liquids, water requires a relatively large energy input to raise its temperature. This large energy input requirement is important for plants because it helps buffer temperature fluctuations.
Latent heat of vaporization
It is the energy needed to separate molecules from the liquid phase and move them into the gas phase at constant temperature—a process that occurs during transpiration. For water at 25°C, the heat of vaporization is 44 kJ mol–1—the highest value known for any liquid. Most of this energy is used to break hydrogen bonds between water molecules.
The high latent heat of vaporization of water enables plants to cool themselves by evaporating water from leaf surfaces, which are prone to heat up because of the radiant input from the sun.
Transpiration is an important component of temperature regulation in plants.
The Cohesive and Adhesive Properties of Water
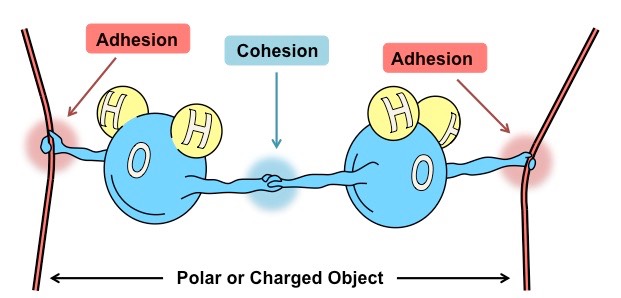
The extensive hydrogen bonding in water also gives rise to the property known as cohesion, the mutual attraction between molecules. A related property, called adhesion, is the attraction of water to a solid phase such as a cell wall or glass surface. Cohesion, adhesion, and surface tension
give rise to a phenomenon known as capillarity, the movement of water along a capillary tube.
Water Has a High Tensile Strength
Cohesion gives water a high tensile strength, defined as the maximum force per unit area that a continuous column of water can withstand before breaking. We do not usually think of liquids as having tensile strength; however, such a property must exist for a water column to be pulled up a
capillary tube.